Abstract
Background: Chimeric antigen t-cell (CAR T) therapy offers high chance of disease remission in B cell lymphomas with a unique one-time treatment. Clinical operations to provide CAR T therapy and autologous hematopoietic cell transplant (auto-HCT) are resource intensive. Due to high acquisition costs for CAR T therapy following approval, its cost effectiveness is questioned (Lin et al, JCO 2019 & Roth et al, J Med Econ 2018). For this reason, many treatment centers are seeking to control treatment delivery costs for auto-HCT and CAR T-cell therapy. It remains unclear what the "real world" clinical and operational cost drivers of CAR T and auto-HCT firms can modify. Furthermore, it is uncertain if cost efficacy analyses done in trials are comparable from the perspective of a treatment center considering adoption of CAR T and/or auto-HCT.
Methods: This is a retrospective single institution cohort study of treated adult patients with relapsed/refractory diffuse large B cell lymphoma (DLBCL) receiving first auto-HCT or commercial CAR T-cell therapy from fiscal year 2018 to 2022. Variable or operational costs (VC) for all services rendered that firms control were recorded from day -7 to day +30 of therapy and compared with descriptive analysis. Therefore, drug acquisition costs of CAR T and bridging therapy were excluded in the analysis. Incremental cost efficacy analysis ratios (ICERs) defined as hospital cost (in USD) incurred for one additional month of disease-free survival obtained. Patient clinical and relapse free survival (RFS) was calculated by Kaplan Meier method. Descriptive analysis and one-way ANOVA was done to assess differences in VCs and ICERs. Regression and multivariate analysis were done to identify potential explanatory cost drivers within results.
Results: 41 patients in auto-HCT and 28 patients in CAR-T cohort met the inclusion criteria. Clinical characteristics are summarized in Table. All patients had relapsed or refractory DLBCL with median line of therapy 2 (range: 1-4) vs 3 (range: 1-5) respectively. The median age was 62 (range: 40-78) vs 64 (range: 18-86) years and approximately 35% of both cohorts were female. Bridging therapy was used in 11 (41%) for CAR T. Median follow up was 19 (range: 4-84) vs 21 (range: 5-38) months in auto-HCT and CAR T therapy. Median RFS was 16 (95% CI: 13-28) vs 16.5 (95% CI: 10-26) months (p > 0.05). Median hospital length of stay was 5 (range: 0-21) versus 7 (range: 0-35).
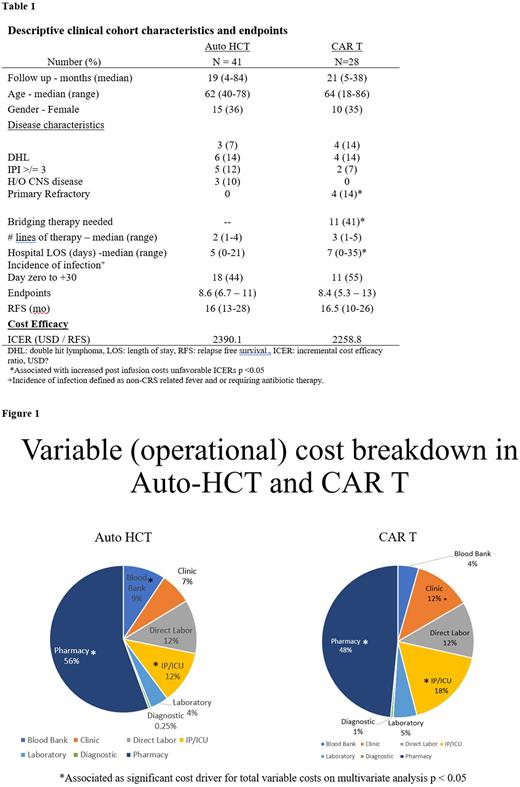
There was no difference in VCs between auto-HCT and CAR T p=0.06. Pharmacy related and inpatient/ICU related capital expenditures represented major cost drivers (Figure 1). However, Grade 2 or 3 neurotoxicity was associated 90th percentile or greater total VCs in CAR T for 6 patients p= 0.01. Average total VCs for CAR T patients reduced over the course of four years by 39% p<0.001. The need for bridging therapy or extended salvage therapy increased 30-day post infusion cost in both cohorts by 8.3% p = 0.002. Cost benefit reflected by ICERs were comparable 2390.1 vs 2258.1 USD per month of RFS gained p= 0.435. The impact of early relapse, prolonged hospital LOS, age, grade 3 infections, neurotoxicity did not negatively affect ICERs for either treatment on multivariate analysis (p=0.33).
Conclusion: In patients with undergoing auto-HCT and CAR T, there is no difference in operational costs that institutions have control over from a healthcare firm perspective. Excluding acquisition costs, pharmacy and inpatient/ICU boarding were major cost drivers. ICER ratios suggest favorable societal outcome in decision analysis for healthcare firms seeking to adopt auto-HCT or CAR T. Further validation with transparent multicenter cost data is needed to inform responsible fiscal adoption and ICER analysis.
Disclosures
Dholaria:BEAM Therapeutics: Consultancy; MJH Biosciences: Honoraria; Gamida Cell: Consultancy; Vanderbilt University Medical Center: Current Employment; Wugen: Research Funding; Janssen: Research Funding; Pfizer: Research Funding; Takeda: Research Funding; Poseida: Research Funding; Molecular Templates: Research Funding; Arivan: Consultancy; Orca Bio: Research Funding; Angiocrine: Research Funding; BMS: Research Funding; MEI Pharma: Research Funding; Jazz Pharmaceuticals: Consultancy. Oluwole:TG Therapeutics: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Janssen: Consultancy; Kite, a Gilead Company: Research Funding; ADC Therapeutics: Consultancy; Curio Science: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal